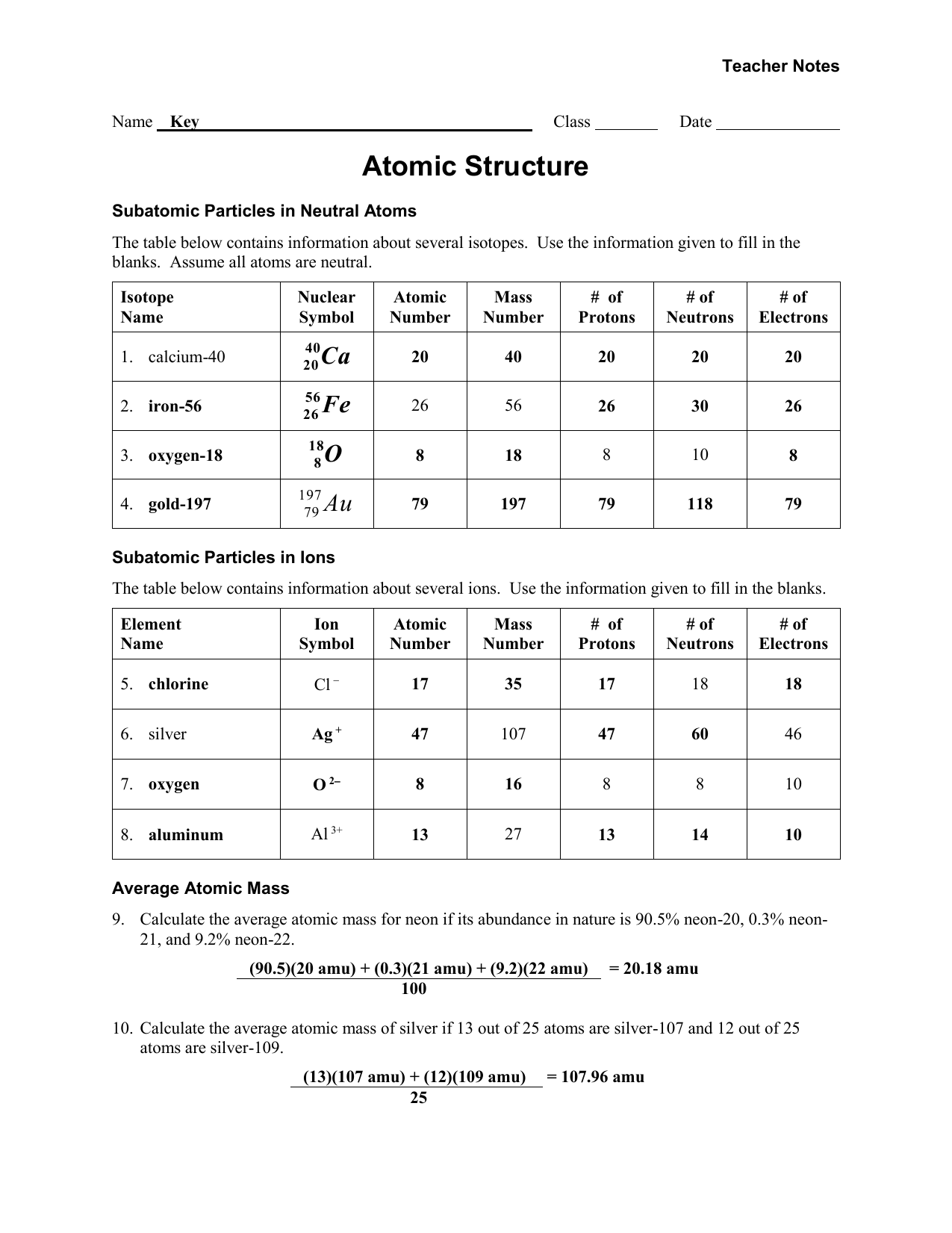
Understanding the basics of atomic structure is crucial in the field of chemistry. It provides the foundation for understanding how elements interact with each other and form compounds. To test your knowledge on this topic, you may have completed a worksheet that challenges you to identify key concepts related to atomic structure. Here, we will provide you with the answer key to help you check your answers and further solidify your understanding.
Atomic structure refers to the arrangement of protons, neutrons, and electrons within an atom. Protons and neutrons are located in the nucleus, while electrons orbit around the nucleus in various energy levels. The number of protons in an atom determines its atomic number, which in turn determines the element’s identity. Understanding these fundamental concepts is essential in chemistry.
1. True or False: Protons have a positive charge.
Answer: True. Protons have a positive charge.
2. How many protons are present in an atom of oxygen (O)?
Answer: An atom of oxygen contains 8 protons.
3. What is the atomic number of an element with 6 protons?
Answer: The atomic number of an element with 6 protons is 6.
4. How many electrons are present in a neutral atom of sulfur (S)?
Answer: A neutral atom of sulfur contains 16 electrons.
5. True or False: Electrons have a negative charge.
Answer: True. Electrons have a negative charge.
In conclusion, understanding atomic structure is essential for anyone studying chemistry. By mastering the basics of protons, neutrons, and electrons, you can navigate the complexities of chemical reactions and bonding. The answer key provided above should help you assess your understanding of atomic structure and guide you in your further studies. Keep practicing and exploring the fascinating world of atoms and elements!