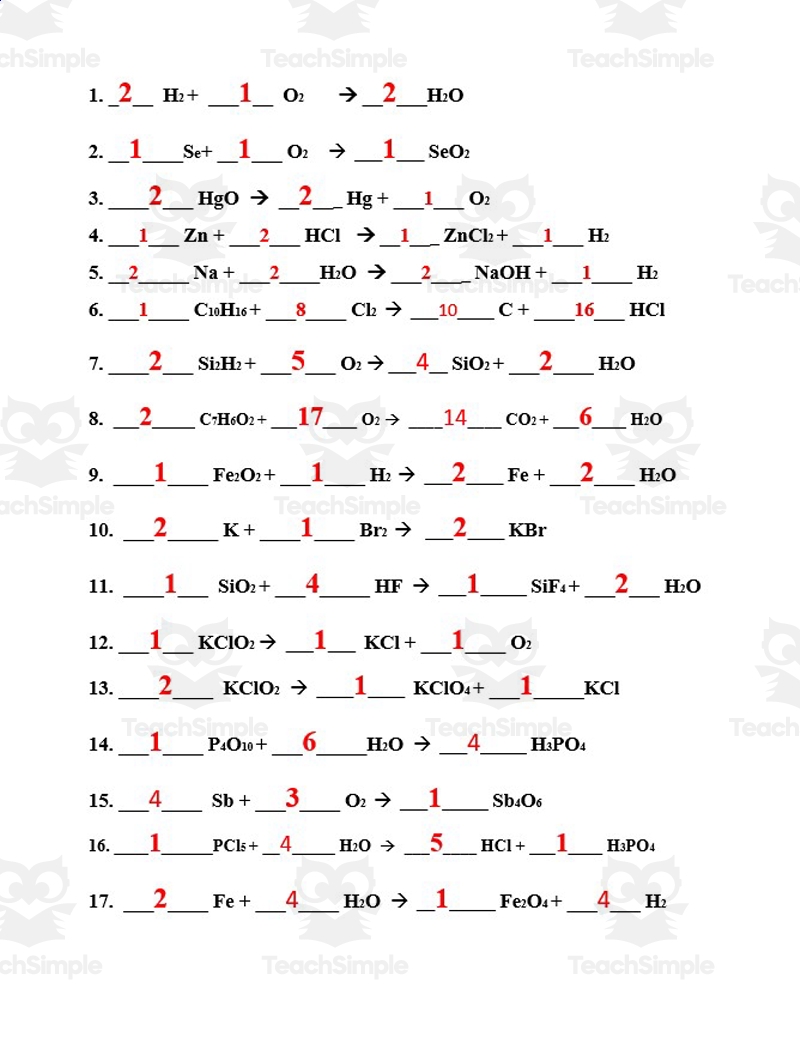
When it comes to chemistry, balancing equations is a fundamental skill that students need to master. It involves ensuring that the number of each type of atom on the reactant side is equal to the number on the product side. This process can be tricky for some, but with practice and guidance, students can become proficient in balancing equations.
One way to practice balancing equations is by using worksheets that provide various chemical reactions for students to balance. These worksheets often come with answer keys that allow students to check their work and see where they may have made mistakes. Having access to an answer key can help students learn from their errors and improve their understanding of the concept.
Answer Key:
1. H2 + O2 → H2O
2. 2H2 + O2 → 2H2O
3. Na + Cl2 → NaCl
4. 2Al + 3CuCl2 → 3Cu + 2AlCl3
5. Fe + O2 → Fe2O3
By working through these equations and checking their answers with the answer key, students can gain confidence in their ability to balance chemical reactions. It’s important for students to understand the principles behind balancing equations, as it is a skill that is used in many areas of chemistry and is essential for understanding chemical reactions.
Practice makes perfect when it comes to balancing equations, so students should not be discouraged if they find it challenging at first. With persistence and the help of answer keys, students can improve their skills and become more proficient in balancing chemical equations. Remember, it’s all about practice and understanding the underlying principles of chemistry!
In conclusion, having an answer key for balancing equations worksheets can be a valuable tool for students to improve their skills and understanding of chemical reactions. By using the answer key to check their work and learn from their mistakes, students can become more confident in their ability to balance equations. With practice and perseverance, students can master this important skill in chemistry.