When it comes to understanding the acidity or basicity of a solution, pH and pOH calculations are essential. pH is a measure of how acidic or basic a solution is, while pOH is a measure of the concentration of hydroxide ions in a solution. By learning how to calculate these values, you can better understand the chemical properties of a solution.
Understanding pH and pOH calculations is crucial in various fields such as chemistry, biology, and environmental science. These calculations help determine the strength of acids and bases, the equilibrium of chemical reactions, and the behavior of ions in a solution. By mastering these calculations, you can make more informed decisions in laboratory experiments and real-world applications.
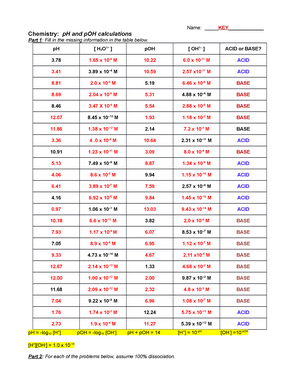
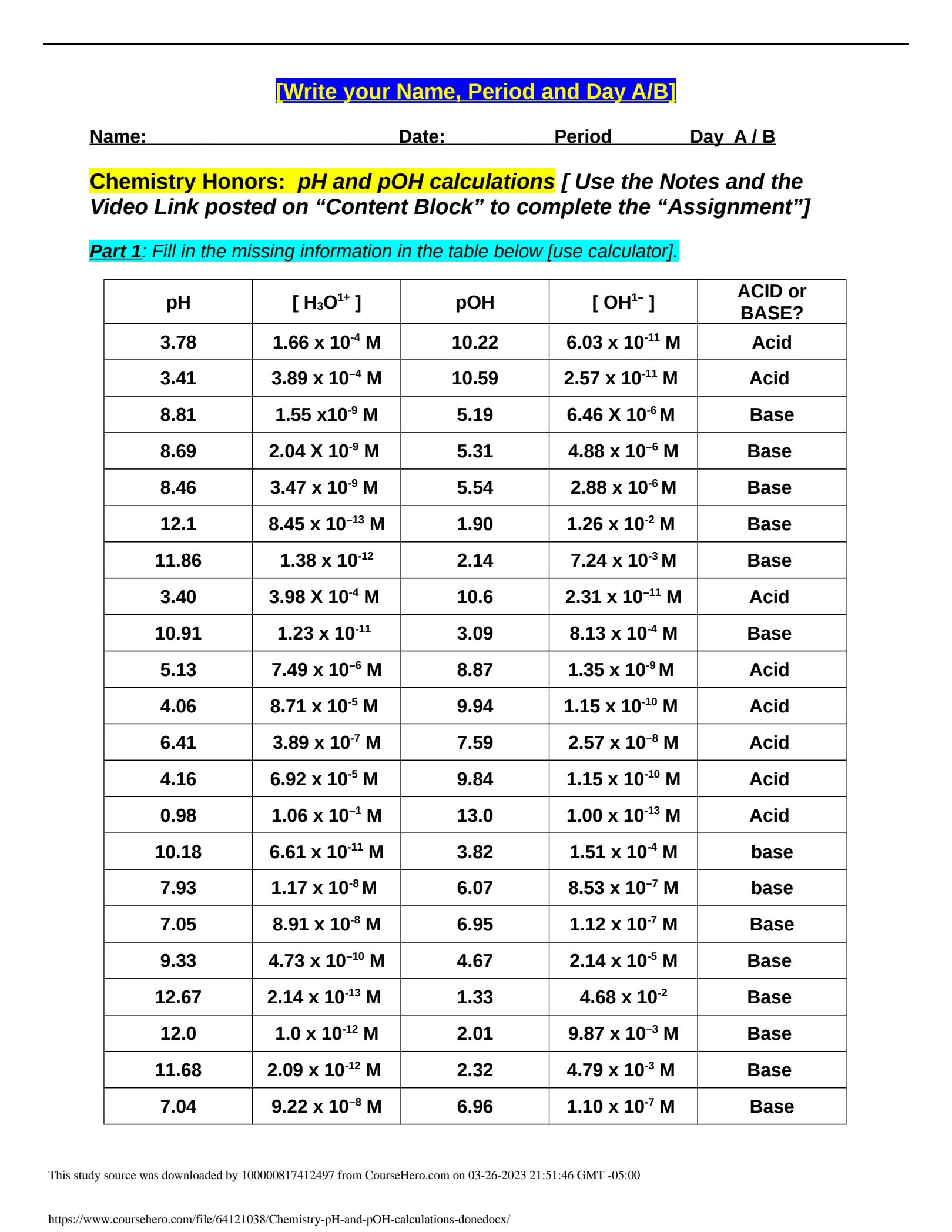
Worksheet pH and pOH Calculations
To calculate pH and pOH, you first need to understand the relationship between hydrogen ions (H+) and hydroxide ions (OH-) in a solution. The pH of a solution is calculated using the formula pH = -log[H+], where [H+] represents the concentration of hydrogen ions in moles per liter. Similarly, the pOH of a solution is calculated using the formula pOH = -log[OH-], where [OH-] represents the concentration of hydroxide ions in moles per liter.
Once you have determined the concentrations of hydrogen and hydroxide ions in a solution, you can calculate the pH and pOH values. For example, if the concentration of hydrogen ions in a solution is 1 x 10^-4 M, the pH would be calculated as pH = -log(1 x 10^-4) = 4. Similarly, if the concentration of hydroxide ions in a solution is 1 x 10^-5 M, the pOH would be calculated as pOH = -log(1 x 10^-5) = 5.
By practicing pH and pOH calculations on worksheets, you can improve your understanding of these concepts and enhance your problem-solving skills. Worksheets typically include various scenarios where you need to calculate the pH or pOH of a solution based on the given concentrations of hydrogen or hydroxide ions. These exercises help reinforce your knowledge and prepare you for more complex calculations in the future.
In conclusion, mastering pH and pOH calculations is essential for anyone studying chemistry or related fields. By understanding how to calculate these values and practicing on worksheets, you can enhance your analytical skills and make more informed decisions in laboratory experiments. Whether you are a student or a professional, knowing how to determine the acidity or basicity of a solution is a valuable skill that can benefit you in various scientific disciplines.